As one of the alternatives for fossil diesel, biodiesel has gained significant attention in recent years. Transesterification is by far the most common method to produce biodiesel oil from plant and animal oils. Due to their limited supply and high cost, food-based biofuels are banned in China. Jatropha oil is an option of great potential for producing biodiesel.
With an aim to optimize the variables for the esterification process of Jatropha oil, Prof. Fang Zhen of XTBG and his colleagues have done many experiments.
In their experiments, Catalytic conversion of un-pretreated Jatropha oil with high-acid value (13.8 mg KOH/g) to biodiesel was studied in ionic liquids (ILs) with metal chlorides. Several commercial ILs were used to catalyze the esterification of oleic acid. It was found that 1-butyl-3-methylimidazolium tosylate ([BMIm][CH3SO3]; a Brønsted acidic IL) had the highest catalytic activity with 93% esterification rate for oleic acid at 140 oC but only 12% biodiesel yield at 120 oC. When FeCl3 was added to [BMIm][CH3SO3], a maximum biodiesel yield of 99.7% was achieved at 120 oC. Because metal ions in ILs supplied Lewis acidic sites, and more of the sites could be provided by trivalent metallic ions than those of bivalent ones. It was also found that the catalytic activity with bivalent metallic ions increased with atomic radius. Mixture of [BMIm][CH3SO3] and FeCl3 was easily separated from products for reuse to avoid producing pollutants.
This “One-step production of biodiesel Jatropha oil with high-acid value in ionic liquids” is now in press in Bioresource Technology.
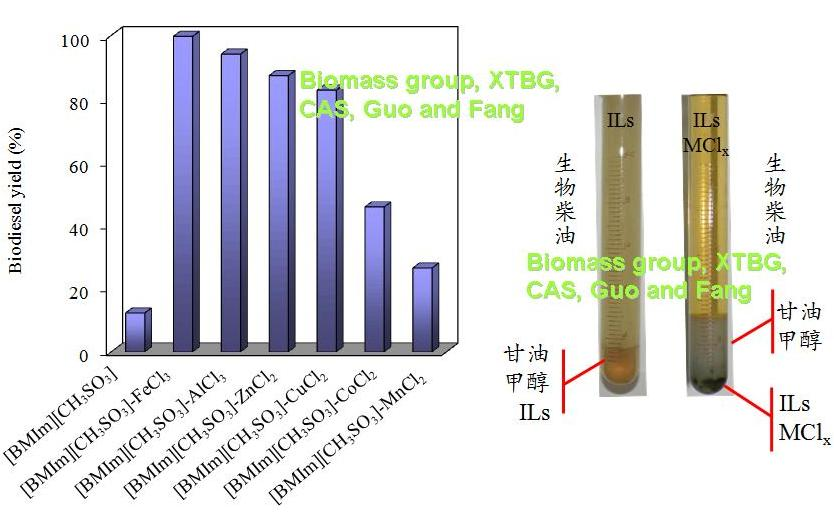
Effect of metal chlorides on transesterification of crude Jatropha oil with methanol

