During the past decades, biodiesel has become more and more important as one of alternative liquid fuels due to the high oil demand and limited petroleum resources. The transesterification of vegetable oils or animal fats to biodiesel by strong basic solution has been widely used in industrial production. However, soaps are usually formed when free fatty acids (FFAs) and water existed in crude oil, resulting in the catalyst deactivation and productivity reduction. Solid base catalyst is much more preferred due to better recovery and recycling. It can catalyze the transesterification of low quality oil or fat in the presence of FFA and water.
Inorganic solid bases are low-cost and easy-to-use heterogeneous catalysts. Reports on metallic salts catalyzed conversion in biodiesel preparation are rare. Dr. GUO Feng of Xishuangbanna Tropical Botanical Garden (XTBG) used calcined sodium silicate (CSS) to catalyze the transesterification reaction for the first time. It catalyzed soybean oil to biodiesel with a yield of almost 100% under the 34 conditions: CSS of 3.0 wt %, a molar ratio of methanol/oil of 7.5:1, reaction time of 60 min and reaction temperature of 60 ºC. The CSS could tolerate 4.0 wt% water or 2.5 wt% FFAs contained in soybean oil.
Based on their previous research, Dr. GUO F. further studied the stability under the direction of Prof. FANG Zhen. A mechanism was then proposed for the transesterification of soybean oil based on the catalyst structures identified by Fourier-Transform Infrared (FT-IR). Density functional theory was also employed to deduce the mechanistic route of transesterification reactions catalyzed with CSS. It was confirmed that alkaline active species were formed from the ion-exchange between CSS and CH3OH. A simple method was found to recover the basic sites to regenerate the catalyst that performed good activity and reproducibility. The study entitled “Transesterification mechanism of soybean oil to biodiesel catalyzed by calcined sodium silicate” is now in press in Fuel .
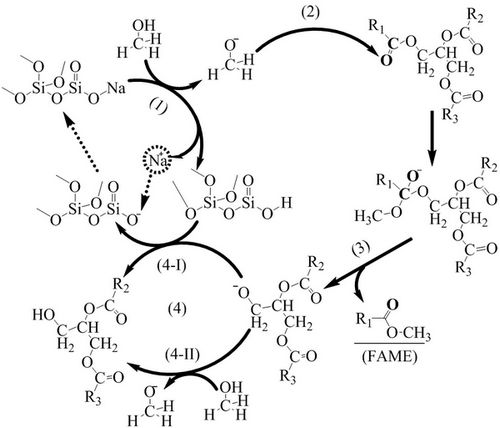
Transesterification Mechanism Catalyzed by the CSS (Image by GUo Feng)
Related publications:
[1] Guo F, Peng ZG, Dai JY, Xiu ZL. Calcined Sodium Silicate as Solid Base Catalyst for Biodiesel Production. Fuel Processing Technology, 2010, 91(3):322-328.
[2] Guo F, Wei NN, Xiu ZL, Fang Z. Transesterification Mechanism of Soybean Oil to Biodiesel Catalyzed by Calcined Sodium Silicate. Fuel, 2011, doi:10.1016/j.fuel.2011.08.064.
[3] Long YD, Guo F, Fang Z, et al. Production of Biodiesel and Lactic Acid from Rapeseed Oil Using Sodium Silicate as Catalyst. Bioresource Technology, 2011, 102(13): 6884-6886.
[4] Guo F, Fang Z. Biodiesel Production with Solid Catalysts, In the Book: Biodiesel, 2011. Edited by Margarita Stoytcheva, InTech. (ISBN 978-953-307-633-1), (ISSN 0192-303X).
[5] Guo F, Fang Z, Long YD. A method of manufacturing biodiesel and lactic acid with silicate as catalyst.Chinese patent: CN201010537935, 5.
[6] Guo F, Xiu ZL, Zhang DJ. Manufacturing biodiesel with calcined sodium silicate. Chinese patent: CN200710159084.3.
[7] Xiu ZL, Guo F, Peng ZG.A method of manufacturing biodiesel with silicate as catalyst. Chinese patent: ZL200710011068.X.

